|
|
RainforestRainforest – Processes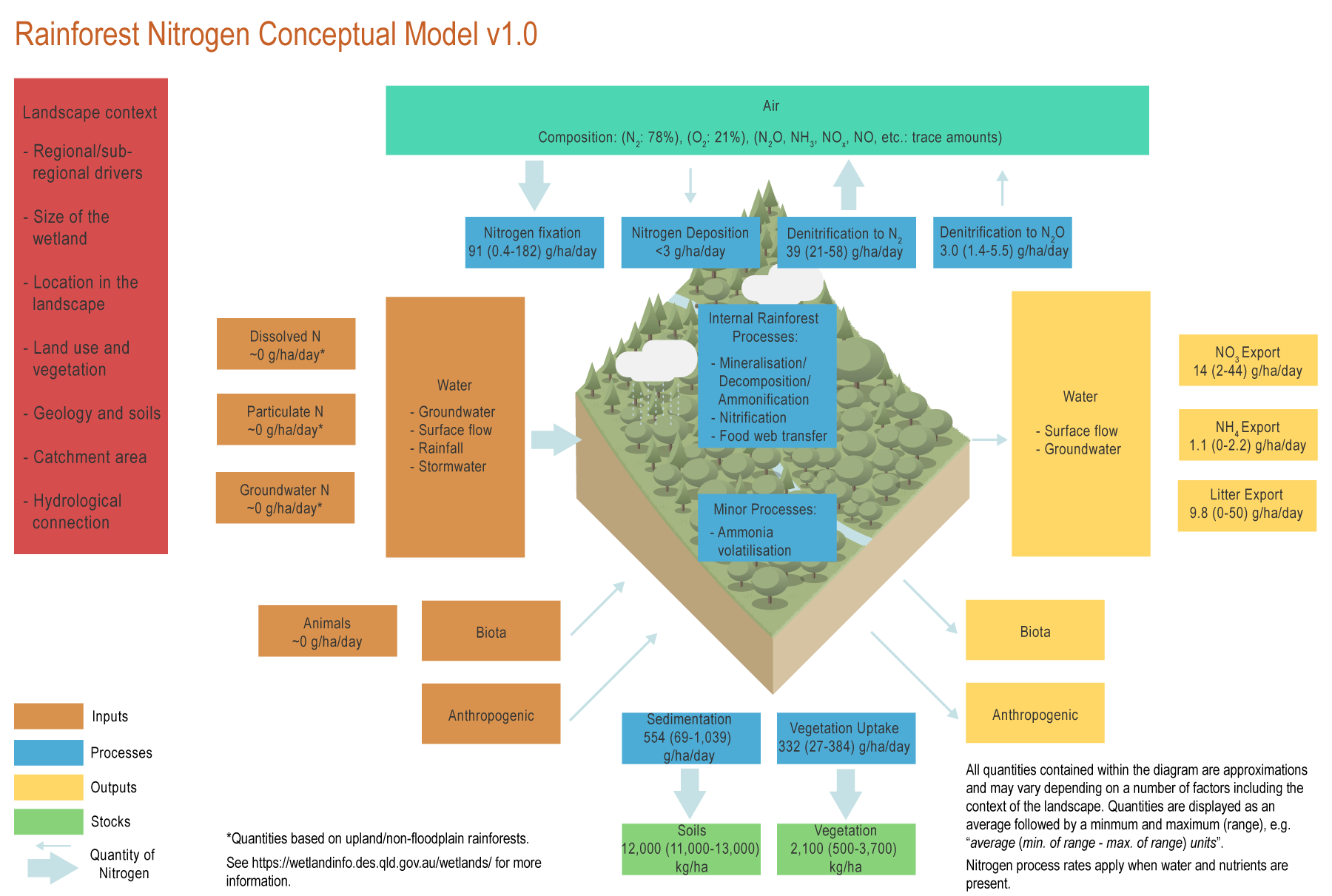
The conceptual models were compiled by researchers in collaboration with a wide range of stakeholders from Natural Resource Management groups, universities and government agencies and based on available scientific information[1]. Click on elements of the model or select from the tabs below Ammonia volatilisationAmmonia volatilisation occurs when pH is in the alkaline range (greater than 8 pH) and where concentrations of urea (derived from animals) are relatively high[12]. Biomass accumulation (vegetation)Rainforest trees can be long-term storages of N, accumulating between 27-384 g of N per hectare of rainforest every day[11]. Mineralisation (Decomposition/Ammonification)Decomposition may be higher through secondary forest succession and is highly regulated by ammonium[13]. Decomposition is closely associated with rates of denitrification and with forest stage (primary, succession or secondary forest), and season, with the highest rates in the wet season[13][8]. DenitrificationDenitrification in rainforests is mostly controlled by the presence or absence of oxygen, as anoxic conditions are required for this process to take place[13]. After oxygen, nitrate availability and carbon are likely sources of limitation for denitrification in rainforests[13]. Denitrification is likely to be an important process in tropical rainforests, accounting for the loss of 24-53% (39 (21-58) g/ha/day)* of N, especially in wetter rainforests[6]. Denitrification may vary depending on successional stage, with the lowest values in mid-succession forests[13]. Denitrification rates are relatively small compared to wetlands, because soils are not always anoxic[13]. Food chain transferIn rainforests most of the carbon and essential nutrients are generally locked up in the living vegetation, dead wood, and decaying leaves. Decaying matter (dead wood and leaf litter) is recycled efficiently by an abundance of decomposing organisms including bacteria, fungi, and termites. These organisms take up nutrients, which are released as wastes when the organisms die, and then taken up by living plants. NitrificationNitrification is seasonal in rainforests in the Great Barrier Reef (GBR) catchments, with higher rates during the wet season[7]. Nitrification is highest when soil moisture is intermediate[7]. Nitrogen deposition from the atmosphereNitrogen can be deposited from the atmosphere to the biosphere as gas, dry deposition and precipitation. However, N deposition is only significant in areas with high industrial activity[10]. In the GBR catchments, N deposition is likely to be below 3 g/ha/day[10]. Nitrogen fixation from the atmosphereNitrogen (N) fixation in rainforests can occur in the roots of legumes, in shallow soils and litterfall, or in epiphytes which can have associated N fixing bacteria[5][2]. N fixation rates average 91 g/ha/day and may range from 0.4-182 g/ha/day, which is large compared to temperate forests[2][9]. Lowest N fixation values are found in mature forests, and highest in secondary forests[3]. SedimentationRainforests can be erosional or accumulate sediment at a rate of 2-2.5 mm per year (554 (69-1,039) g/ha/day)*[1]. Unlike wetlands, most of the accumulation of N is derived from locally produced litter and not from external sources[4]. The accumulated sediment can be rapidly decomposed[13][8]. *Nitrogen quantities are displayed as an average followed by a minimum and maximum (range), e.g. “average (min. of range - max. of range) units”. References
Last updated: 2 August 2021 This page should be cited as: Department of the Environment, Tourism, Science and Innovation, Queensland (2021) Rainforest – Processes, WetlandInfo website, accessed 15 December 2025. Available at: https://wetlandinfo.detsi.qld.gov.au/wetlands/ecology/processes-systems/nitrogen-concept-model/rainforest/processes.html |

 — Department of the Environment, Tourism, Science and Innovation
— Department of the Environment, Tourism, Science and Innovation

