|
|
Nitrogen processes and cycleNitrogen processes and cycle – Chemical forms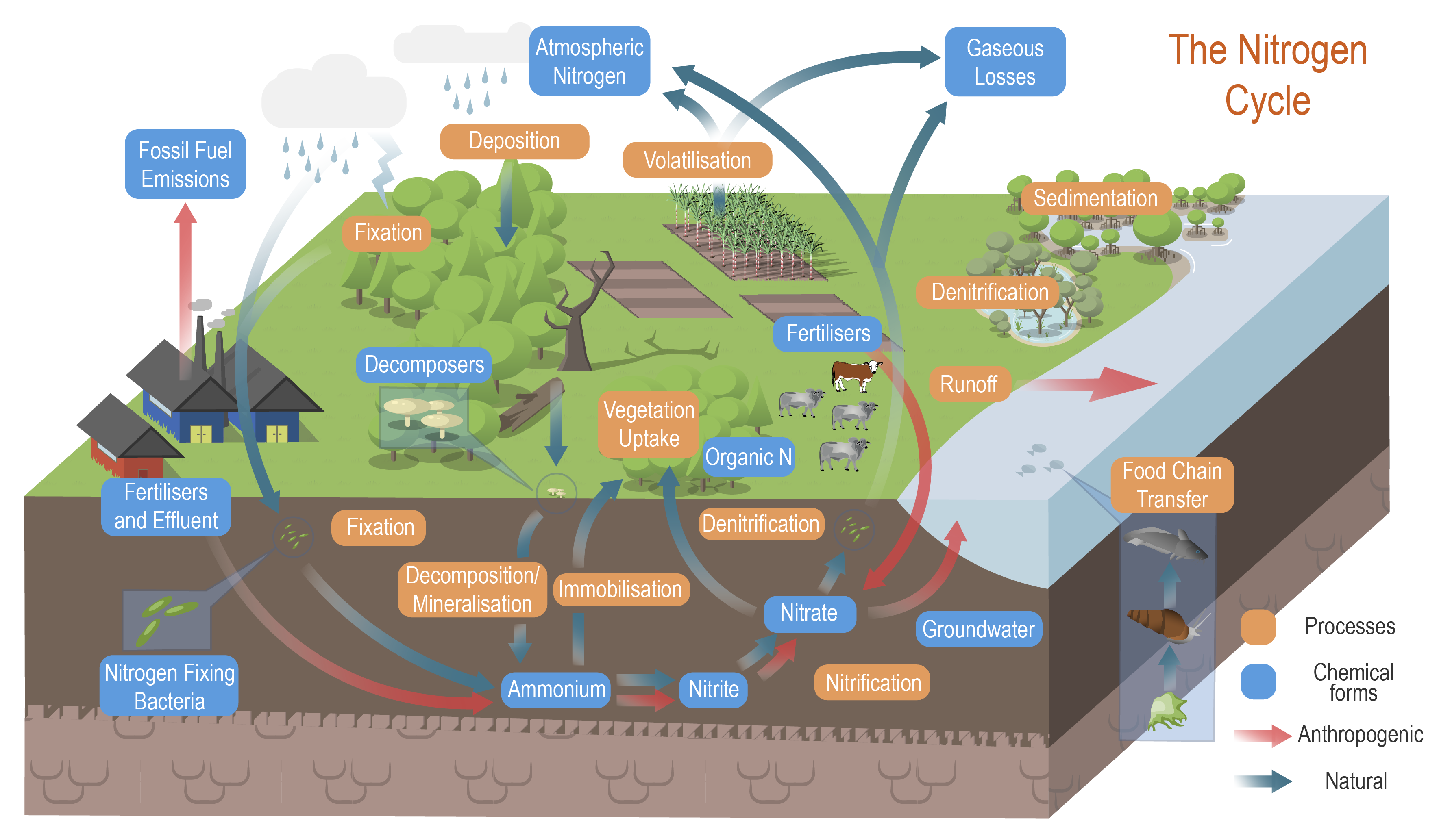
The conceptual models were compiled by researchers in collaboration with a wide range of stakeholders from Natural Resource Management groups, universities and government agencies and based on available scientific information[1]. Click on elements of the model or select from the tabs below Nitrogen exists in many different forms in the atmosphere, water, soils and biota. The form nitrogen takes depends on physical, chemical and biological conditions. Ammonia (NH3)Ammonia is a compound that usually occurs as a gas, vapor, or liquid[10]. It is colourless and readily dissolves in water. It is present in soil under alkaline conditions. Ammonium (NH4+)Ammonium is a positively charged ion (or cation), formed by the addition of a hydrogen ion (H+) to ammonia. Ammonium is soluble in water, although it is also electrochemically attracted to negatively charged surfaces associated with clays and organic matter in soil, and is therefore not as mobile as nitrate[7]. Plants that are adapted to acidic soils can use ammonium as their source of nitrogen, although most species on non-acidic soils can only use nitrate. Dissolved inorganic nitrogen (DIN)DIN is dissolved inorganic nitrogen. It is comprised of nitrate, nitrite and ammonium[5]. Dissolved organic nitrogen (DON)DON is dissolved organic nitrogen that passes through a 0.45 μm or 0.2 μm membrane filter[10][1]. The compounds originate from photosynthetic organisms (algae and plants), excretion of nitrogenous waste by animals, leachate from soil, sewage discharge, and atmospheric deposition. Nitrate (NO3-)Nitrate is a negatively charged ion (or anion), and is highly soluble in water[6]. Nitrate is the preferred form of nitrogen for uptake by most terrestrial species of plants[7]. If nitrate is not assimilated by plants or microorganisms, it is readily leached from soils. Nitric oxide gas (NO)Nitric oxide (NO) is a gas that is emitted to the atmosphere mostly as a result of combustion[6]. NO is generated by two mechanisms[10]:
Nitrite (NO2-)Nitrite is a short term intermediate molecule in oxidative and reductive processes such as nitrification and denitrification. It also serves as a constituent for the anammox process[11]. Nitrogen dioxide gas (NO2) and its productsThe term nitrogen oxides (NOx) describes a mixture of nitric oxide (NO) and nitrogen dioxide NO2), which are gases produced from natural sources, motor vehicles and other fuel burning processes[6]. Nitric oxide is colourless and is oxidised in the atmosphere to form nitrogen dioxide. Nitrogen dioxide has an odour, and is an acidic and highly corrosive gas. NO is oxidized in the atmosphere to HNO3 which can combine with NH3 to form ammonium nitrate salt. This particle then deposits on the earth’s surface[3]. Nitrogen dioxide also reacts in the atmosphere to form nitric acid, contributing to the problem of acid rain[8]. Nitrogen gas (N2), DinitrogenDinitrogen is an unreactive and stable gas, held together by a relatively strong, triple bond. About 78% of the volume of Earth's atmosphere consists of dinitrogen[6], but because of its almost inert character few organisms can directly use this gas in their nutrition. N2 must be "fixed" into other forms before it can be assimilated by most organisms. Sometimes this molecule is less precisely called "nitrogen," although that term should be restricted to nitrogen atoms. Nitrous oxide gas (N2O)Nitrous oxide gas N2O is a relatively unreactive gas and persistent in the atmosphere[10]. It is also, a potent greenhouse gas. However, N2O is very slowly oxidized to NOx gases under the influence of sunlight and atmospheric catalysts. The major source of atmospheric N2O is denitrification occurring in wet, nitrate-rich soils. Organic nitrogenOrganic nitrogen describes a nitrogen compound that had its origin in living material[9]. The nitrogen in proteins and urea (a byproduct of protein digestion) is organic nitrogen. Particulate inorganic nitrogen (PIN)PIN is particulate inorganic nitrogen[12]. This includes soluble nitrate and ammonium in the interstitial pore water, and adsorbed ammonium. Particulate organic nitrogen (PON)PON is particulate organic nitrogen[12]. It is found in eroded sediment and includes a light fraction, constituted by a mixture of the remains of plants, animals and microorganisms at various stages of decomposition[2] and a heavy fraction which is attached to fine mineral sediment particles (i.e. silt and clay) through chemical bonds[4]. References
Last updated: 9 August 2021 This page should be cited as: Department of Environment, Science and Innovation, Queensland (2021) Nitrogen processes and cycle – Chemical forms, WetlandInfo website, accessed 8 May 2025. Available at: https://wetlandinfo.des.qld.gov.au/wetlands/ecology/processes-systems/nitrogen-concept-model/physical-chem.html |

 — Department of the Environment, Tourism, Science and Innovation
— Department of the Environment, Tourism, Science and Innovation

