|
|
Carbon processes and cycle
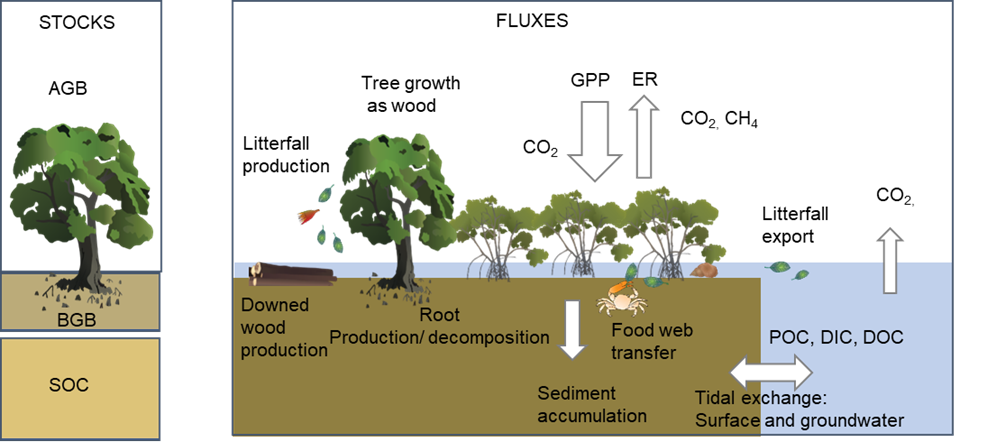
Carbon is one of the most common elements on earth and provides structure for all living organisms. Plants and algae get carbon from the atmosphere as carbon dioxide (CO2) and fix it through photosynthesis to form sugars and plant biomass. Animals can then consume these plants and obtain food from them. Some of the carbon in wetland plants can be preserved in the wetlands’ soil, which is low in oxygen and has low rates of decomposition. This is why wetlands can reduce the levels of carbon dioxide in the atmosphere, particularly with accumulation of peat and through blue carbon ecosystems, reducing greenhouse gases and the effects of climate change. The amount and movement of carbon in wetlands has numerous implications, such as affecting fisheries productivity, controlling resilience to erosion, and mitigating climate change. The carbon cycle refers to the many processes by which natural systems absorb and emit carbon. Plants and animals use carbon to build their cell structures. Carbon can also be deposited in soils. This is how carbon is absorbed or ‘sequestered’. Stored carbon can be released or emitted through natural respiration or when cell structures decompose, when plants are burnt, or in the case of soil carbon, when soils are disturbed. Prior to the Industrial Revolution, the carbon cycle processes of emitting and sequestering were generally balanced with vast amounts of carbon being trapped over many millennia in highly condensed forms such as coal, oil and natural gas, also known as fossil fuels. Since the Industrial Revolution, the carbon content of fossil fuels is being released or emitted as those fuels are burnt for generating energy and manufacturing. Also, the natural sequestering processes, especially those performed by plants, are being disrupted as land is cleared for human needs. These carbon emissions are a major driver of the greenhouse effect and human-induced climate change. The carbon fixed by the plants in wetlands can be stored as plant biomass or soil organic matter, transformed though consumption, or exported through flooding (see Figure 1 below)[2][1]. Water also triggers the release of organic carbon from leaf litter, sticks and grasses on the wetland flood and dissolves into the water. Bacteria feed on the dissolved carbon and form 'bio-film' which is then fed on by larger organisms. Over time, some of the organic carbon can decompose to form peat. Peat swamps also play an important role in carbon storage, although are rare in Australia. Riparian vegetation also drops into streams, forming timber snags that are important shelters for fish, or washing up on the beach as wrack that provides food and shelter for beach organisms. Carbon cycle processesThere are many processes in wetlands that incorporate carbon and are part of the carbon cycle, such as:
Figure 1 - Carbon fluxes in tidal wetlands. Stocks are the carbon stored in the long-term (carbon density per area) and fluxes are processes that involve the conversion of one form of carbon to the other (carbon per area per unit of time). GPP is gross primary productivity (or CO2 fixed), ER is ecosystem respiration (CO2 and CH4 released); POC is particulate organic carbon (e.g., leaves), DOC is dissolved organic carbon (e.g. amino acids) and DIC is dissolved inorganic carbon (e.g. carbonates). Carbon and the oceansAtmospheric carbon forms carbonic acid when combined with water and falls to the surface as rain. This contributes to chemical weathering of rocks, which results in the transport of different elements and ions to the ocean. In the ocean, the calcium ions combine with bicarbonate ions to form calcium carbonate - used by shell-building organisms (oysters, mussels), corals and plankton (which absorb carbon into their cells). Over time, the remnants of these organisms cement together and turn into rock (such as limestone). Chemical processes regulate this cycle between ocean, land, and atmosphere. Around 30% of carbon dioxide in the atmosphere is diffused into the ocean through chemical exchange. Carbonic acid is now slightly changing the pH of the ocean. The reaction in the ocean between carbonic acid and carbonate ions can impact the carbonate ions available for shell building organisms, and the acidity of the ocean weakens marine organisms shells. Carbon in the oceans increases the growth of phytoplankton and ocean plants (such as seagrass). Carbonic acid reacts with, and dissolves, biogenic carbonates – see Intertidal and Subtidal Substrate Composition. Links and referencesPages under this sectionReferences
Last updated: 10 September 2020 This page should be cited as: Department of Environment, Science and Innovation, Queensland (2020) Carbon processes and cycle, WetlandInfo website, accessed 8 May 2025. Available at: https://wetlandinfo.des.qld.gov.au/wetlands/ecology/processes-systems/carbon-cycle/ |

 — Department of the Environment, Tourism, Science and Innovation
— Department of the Environment, Tourism, Science and Innovation