|
|
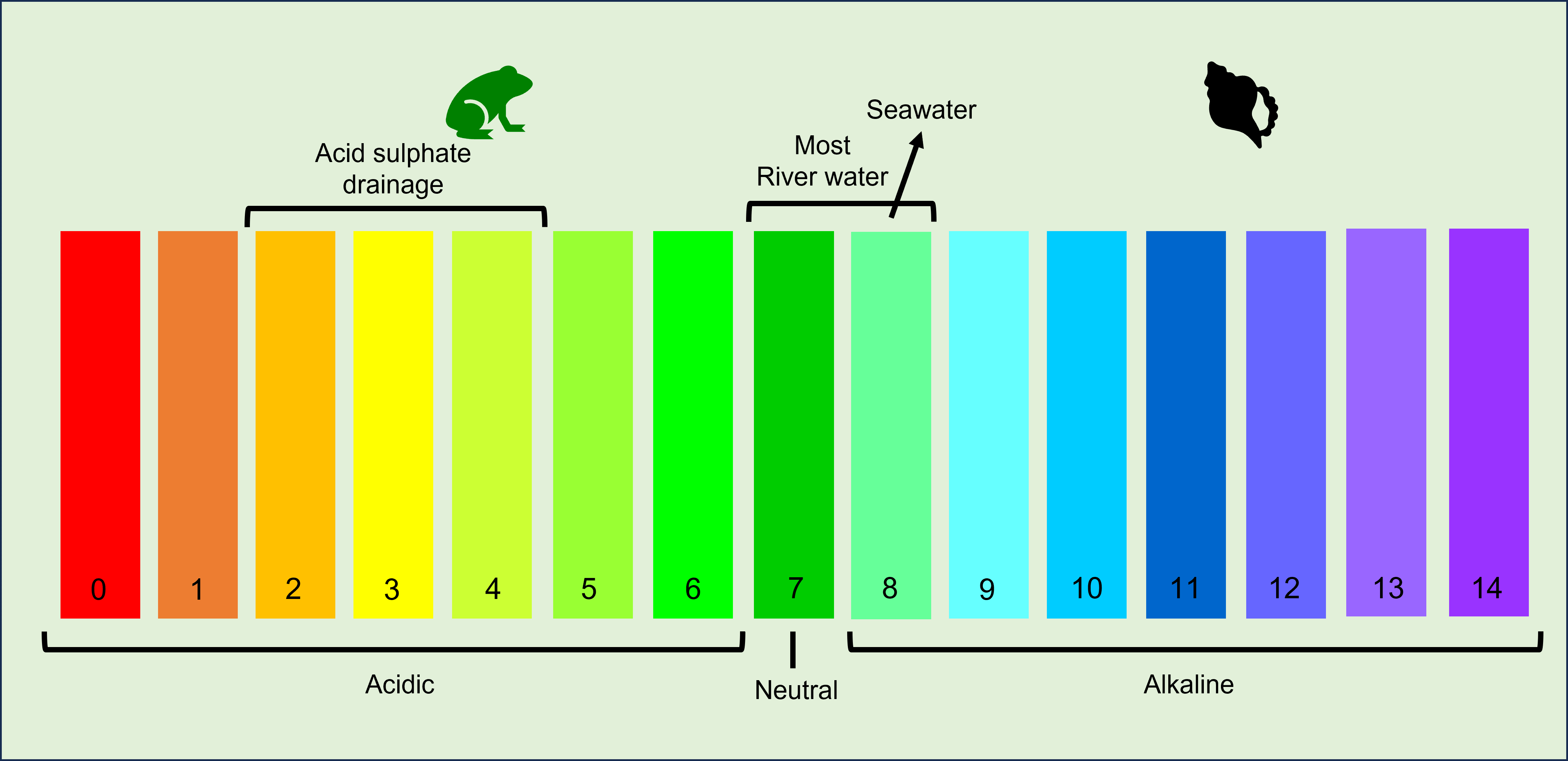
pH (water)The pH of soil or water refers to its acidity and the scale ranges from 0 (highly acidic, acid), through neutral (7) to 14 (highly alkaline, base). The pH of most biological fluids are in the range pH 6 to pH 8. Pure distilled water is pH 7. Most natural freshwaters have a pH range of 6.5 – 8, and the pH of most marine waters is close to 8.2[1]. Wetland plants and animals have adapted to defined pH regimes - some are adapted to high pH (alkaline) and some to low pH (acidic) systems. Naturally occurring low pH is sometimes caused by the breakdown of plant matter by bacteria and fungi and the build up of organic acids, combined with the low buffering potential of soils. Known as humic waters, these have naturally low pH of 5.0-6.0, and as low as 4.5 and are often found in sandy environments. Some specialised plants and animals that live in naturally acidic environments such as the acid frogs and acid fish of the Wallum systems of South east Queensland. Aside from these environments, extreme pH levels can stress or harm aquatic life, impacting life processes such as reproduction, growth, photosynthesis and nutrient uptake[2]. Quick facts
pH affects and drives many chemical reactions. The pH of most living cells is close to 7. A slight change in pH can be very harmful because the chemical processes of the cell are sensitive to concentrations of hydrogen and hydroxide ions. A change of pH in water may trigger algal blooms, the loss of sensitive species, leaching of toxic metals, as they become more soluble in lower pH water, and the loss of biota in mass mortality events. The availability of essential nutrients like phosphorus and nitrogen are influenced by pH levels. The oceans also absorb carbon dioxide (CO2) from the atmosphere, which contributes to ocean acidification. Salinity in water can increase pH, increasing alkalinity. While it can vary, rain generally has a pH of about 5.6 which is slighly acidic. Acid rain, or precipitation, refers to rain/snow/fog that is less than pH 5.6. The cause of acid rain varies, but is largely related to the presence of sulfur oxides and nitrogen oxide gases in the air that react with water in the air to form strong acids. These acids then fall as precipitation. The pH scale, diagram by Queensland Government Another major source of acid can result from the disturbance of acid sulfate soils, which are common along the coast of Queensland and can have significant impacts if not managed correctly. Buffers and buffering capacityMost waters have some capacity to buffer changes in pH. Buffering relates to the ability of a solution or substrate to resist pH changes when excess acidic or alkaline components are added. The presence of buffers helps animals and humans to resist changes in pH. The buffer accepts hydrogen ions when in excess, and donates them when they have been depleted. One of the buffers that contribute to pH stability in blood is carbonic acid (h2CO3). Biocarbonate ions (HCO3-) in water play a buffering role which help prevent rapid pH fluctuations. In areas with low buffering capacity, pH can change significantly during the day – particulary if there are high rates of primary production. High phytoplankton growth uses dissolved CO2 faster than it can be replaced from the atmosphere, impacting the buffering capacity (the CO2 HCO2 equilibrium) of the water and resulting in an increase in pH as the day progresses. During the night, productivity is reduced and CO2 is replenished which drops the pH down again. What influences pH?There are a range of factors which can impact on the pH of water including: Geology and substrate: The underlying geology and soil can contribute to the pH regime of a waterbody and wetland. For example, limestone is largely comprised of calcium carbonate and wetlands in these areas will have a higher pH. Acid sulfate soils can release sulfuric acid into the surrounding environment. Organic matter and biological activity: Decaying organic matter can release CO2 into the water which can form carbonic acid and reduce pH. Primary production (photosynthesis and respiration) from aquatic biota can influence the pH with the consumption of CO2. Nutrient levels and changes: Elevated nutrient levels, particularly phosphates and nitrates from agricultural runoff can promote the growth of algae. As above, algae can influence pH through high rates of photosynthesis and respiration. Temperature: Higher temperatures can lead to the absorption of more gases like CO2, lowering the pH of a waterway through the production of carbonic acid. Links and referencesPressures - pH (Lacustrine and palustrine) ANZECC-ARMCANZ Water Quality Guidelines. References
Last updated: 23 October 2023 This page should be cited as: Department of Environment, Science and Innovation, Queensland (2023) pH (water), WetlandInfo website, accessed 30 August 2024. Available at: https://wetlandinfo.des.qld.gov.au/wetlands/ecology/components/water-chemical/ph-water/ |

 — Department of Environment, Science and Innovation
— Department of Environment, Science and Innovation