|
|
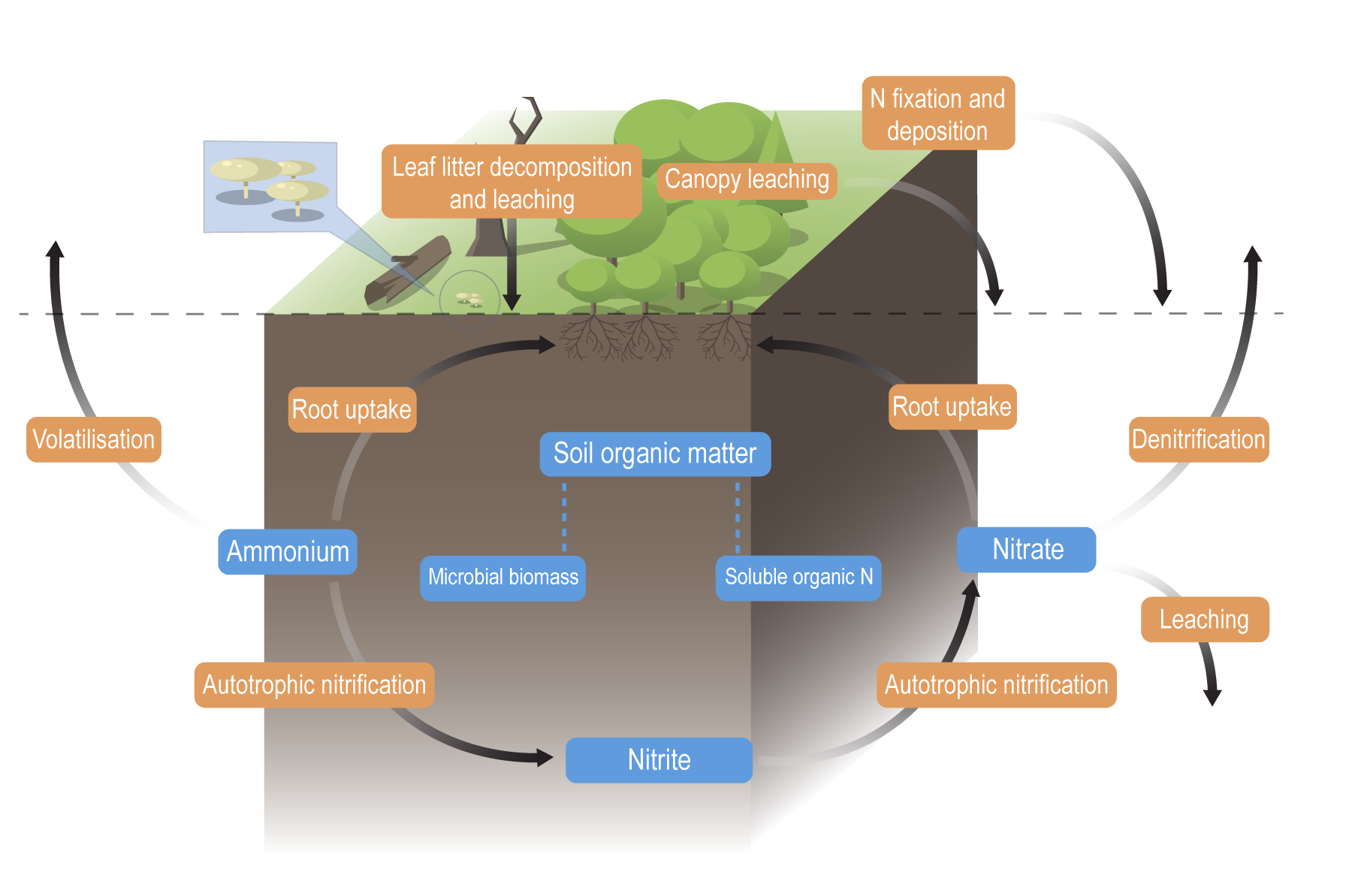
Nitrogen processes and cycleNitrogen processes and cycle – Processes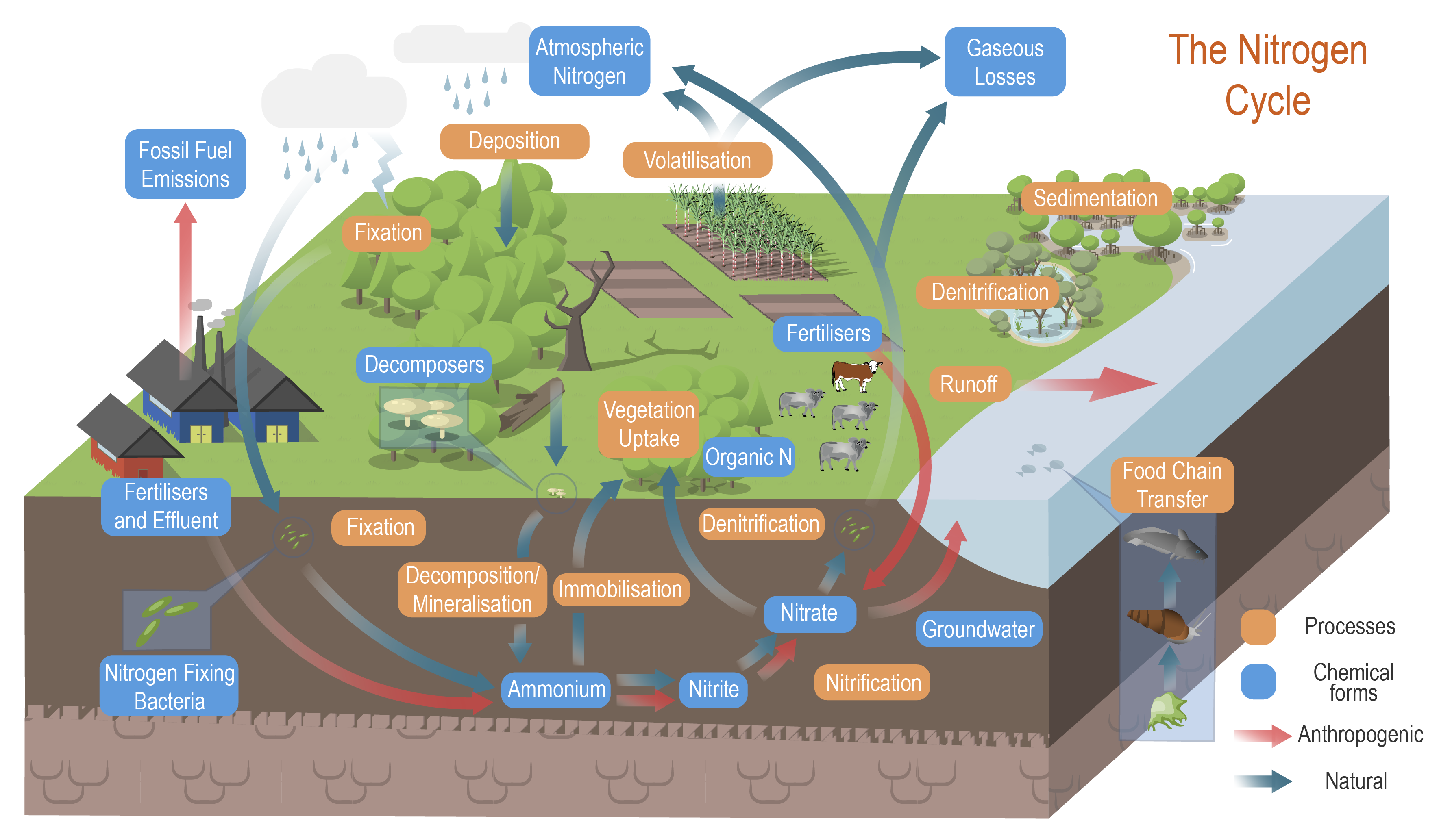
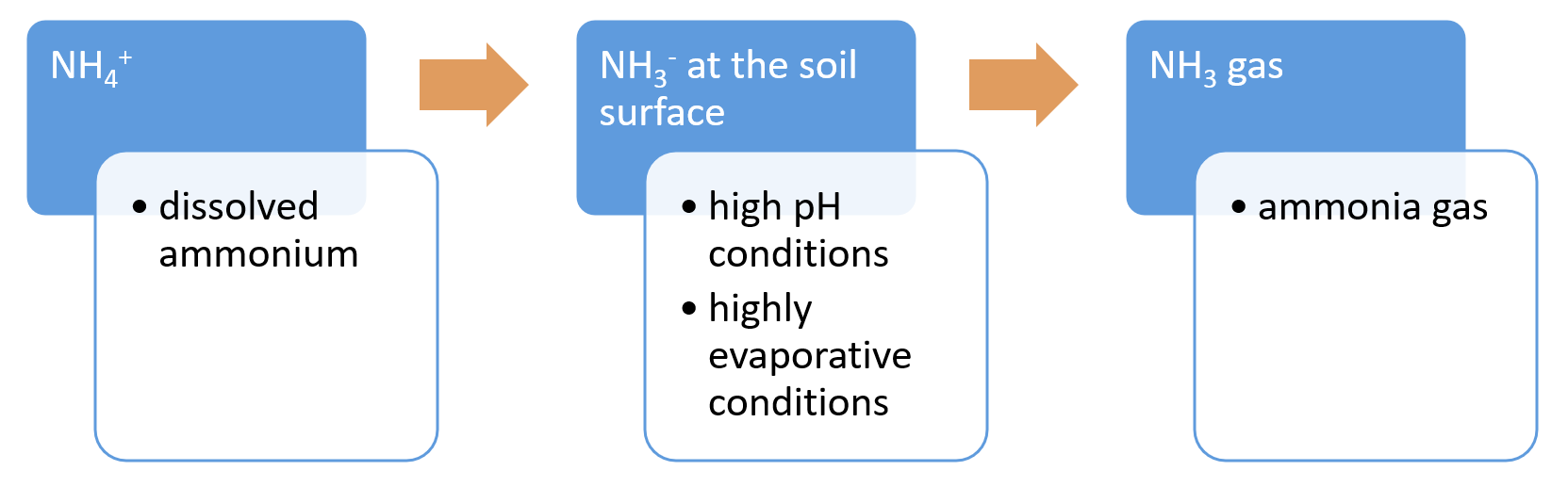
The conceptual models were compiled by researchers in collaboration with a wide range of stakeholders from Natural Resource Management groups, universities and government agencies and based on available scientific information[1]. Click on elements of the model or select from the tabs below Nitrogen (N) cycles through the environment via a wide range of physical and biological processes. These processes are influenced by environmental conditions such as hydrology, carbon source, oxygen availability, pH, soil moisture, salinity, presence of microbes, and temperature. Ammonia volatilisationAmmonia volatilisation is the loss of N through the conversion of ammonium to ammonia gas, which is released to the atmosphere[6]. The volatilisation losses increase at higher soil pH and conditions that favour evaporation (e.g. hot and windy)[21].
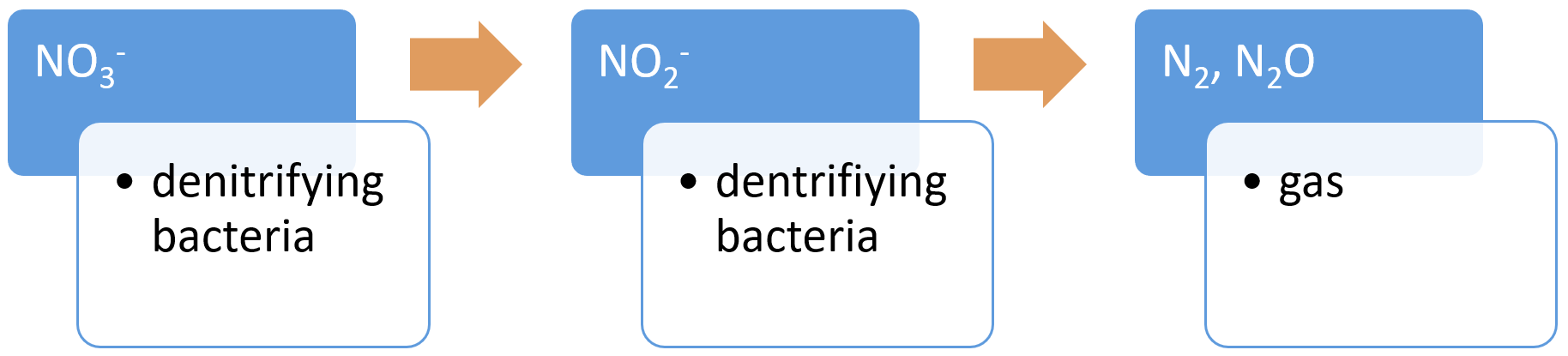
AnammoxAnammox is the process of anaerobic ammonium oxidation. This process involves the conversion of nitrate and ammonium to N2 gas by bacteria of the phylum Planctomycetes. Anammox requires anaerobic conditions, basic pH, and the presence of both ammonium and nitrite, and high carbon[11][22]. Anammox also results in the removal of nitrogen from the system in the form of nitrogen gas[4]. Biomass accumulation (vegetation uptake)Biomass accumulation, or vegetation uptake, is the build-up of nitrogen as plant material (biomass) through grass, herb, sedge, shrub, aquatic vegetation, and tree growth. Trees can accumulate and store carbon and nutrients for long time periods[2]. However, many types of wetland vegetation may return nitrogen and other nutrients to the soil when the plant dies and is rapidly decomposed, resulting in a ‘recycling’ of nitrogen. Higher nitrogen accumulation as plant biomass will occur in wetlands with warm and wet conditions. Areas of excessive weed growth may be an indication of high nitrogen accumulation[14]. DenitrificationThe denitrification process is especially active in water-logged anaerobic soils. The action of these bacteria uses nitrates and carbon for metabolism and produces free atmospheric nitrogen (N2), and other gaseous forms of N, such as nitric oxide (NO), and nitrous oxide (N2O)[19]. Export of nitrogen as N2O is likely to occur as a result of incomplete denitrification and nitrification, but generally accounts for less than 1% of nitrogen removed from denitrification[3]. In anaerobic conditions, if nitrate concentrations are high, denitrification occurs but only if hydroxides of iron and manganese are present[15], or through anaerobic methane oxidation coupled with denitrification[18]. The denitrification process is one of the key removal pathways of nitrogen by wetlands[1].
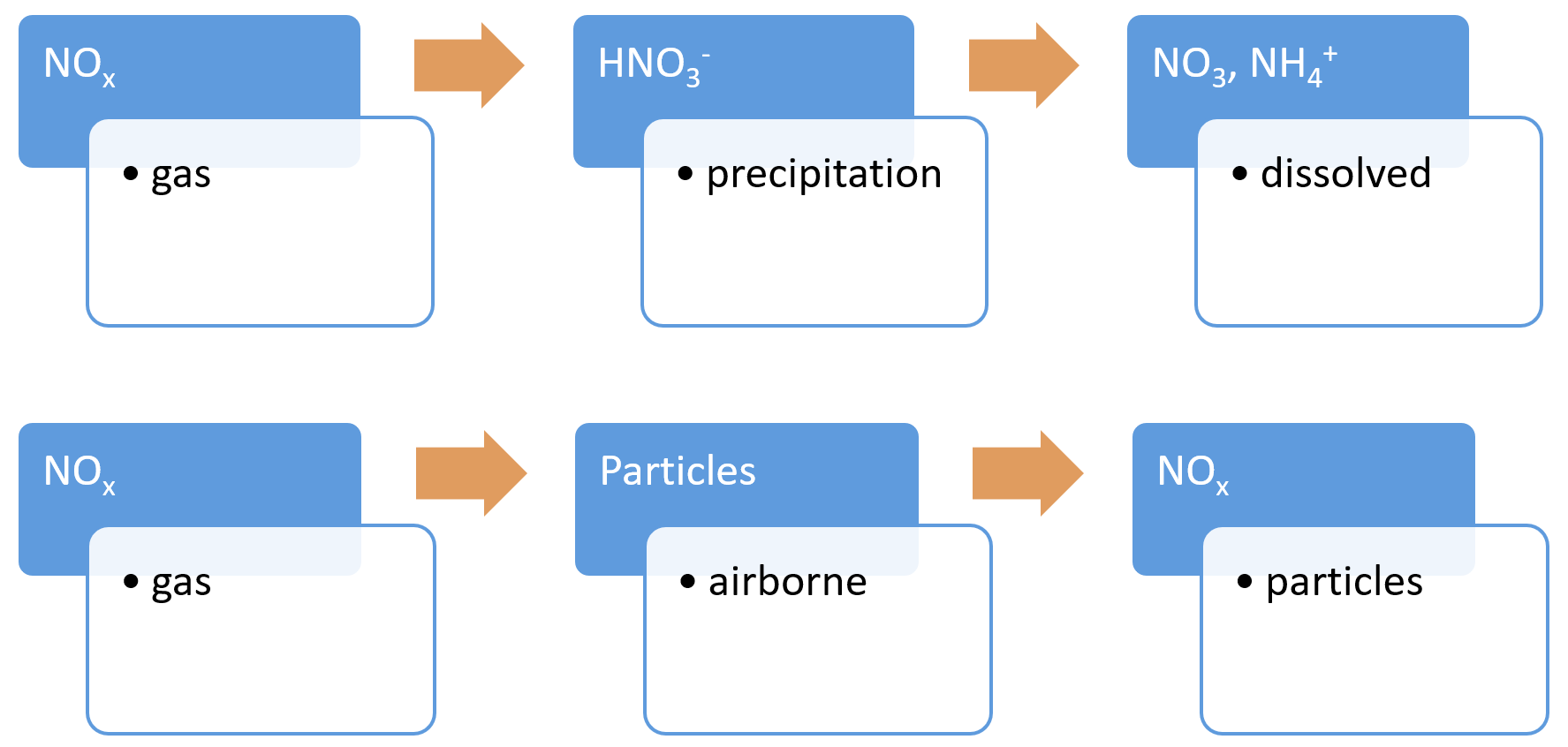
Deposition (nitrogen)Nitrogen deposition is the input of reactive nitrogen from the atmosphere to the biosphere. It can be either dry deposition (gases and dust) or wet deposition (precipitation)[17]. Increased reactive nitrogen deposition in some regions is a consequence of global emissions of oxidised forms of nitrogen (nitric oxide (NO)), nitric acid (NO3) and nitrogen dioxide (NO2) – often referred to as nitrogen oxides (NOx)) from fossil fuel combustion, and reduced N (NHx) from agricultural sources[17].
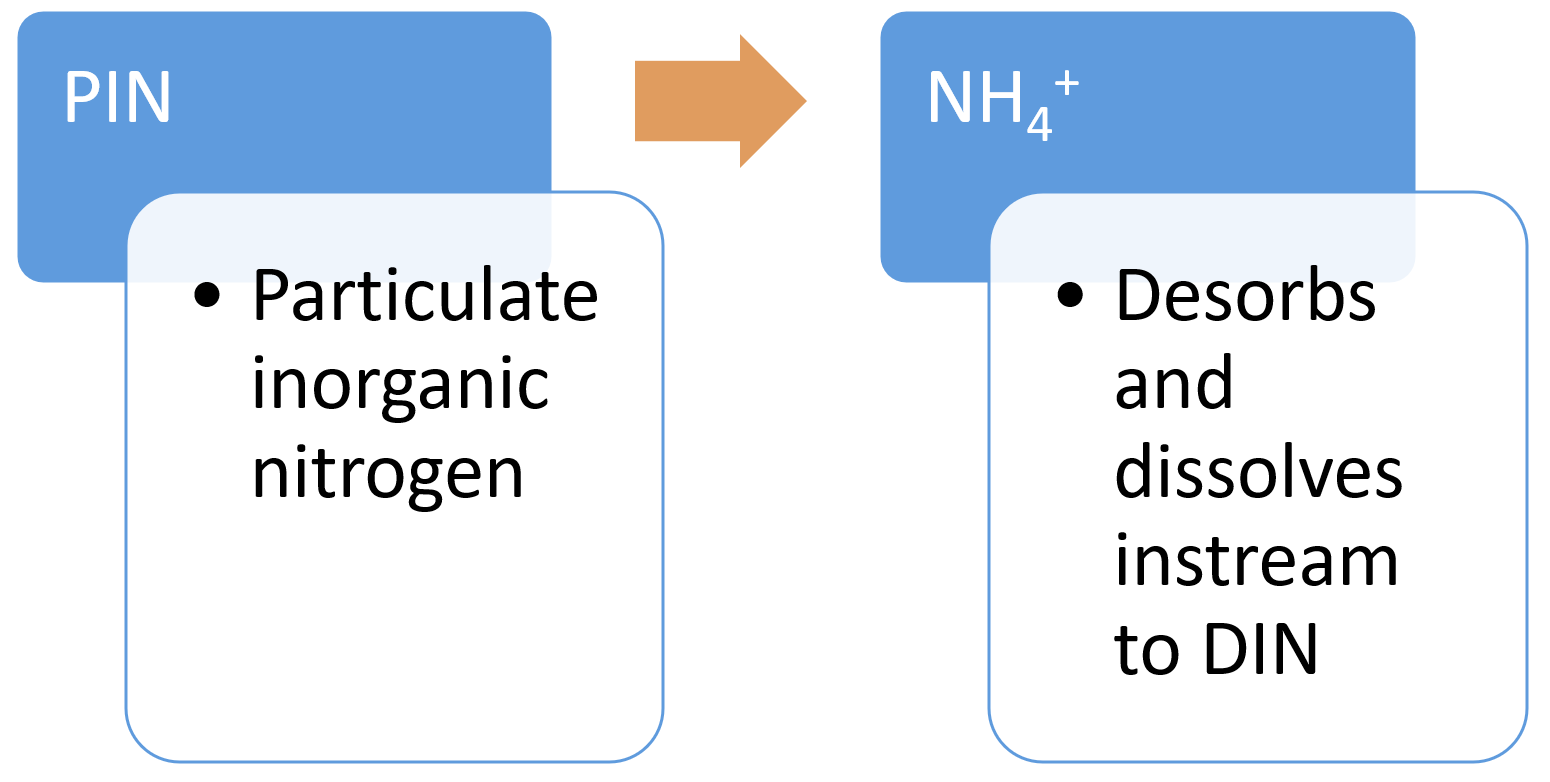
Desorption of particulate inorganic nitrogenDesorption of PIN (particulate inorganic nitrogen) is a physico-chemical process in which the ammonium (NH4+-N) adsorbed to the negatively charged silt and clay particles in sediment is desorbed (becomes soluble) through cation exchange processes (e.g. exchange of ammonium with sodium or magnesium) in water[7]. This often occurs when terrestrial sediment enters saline water in estuaries.
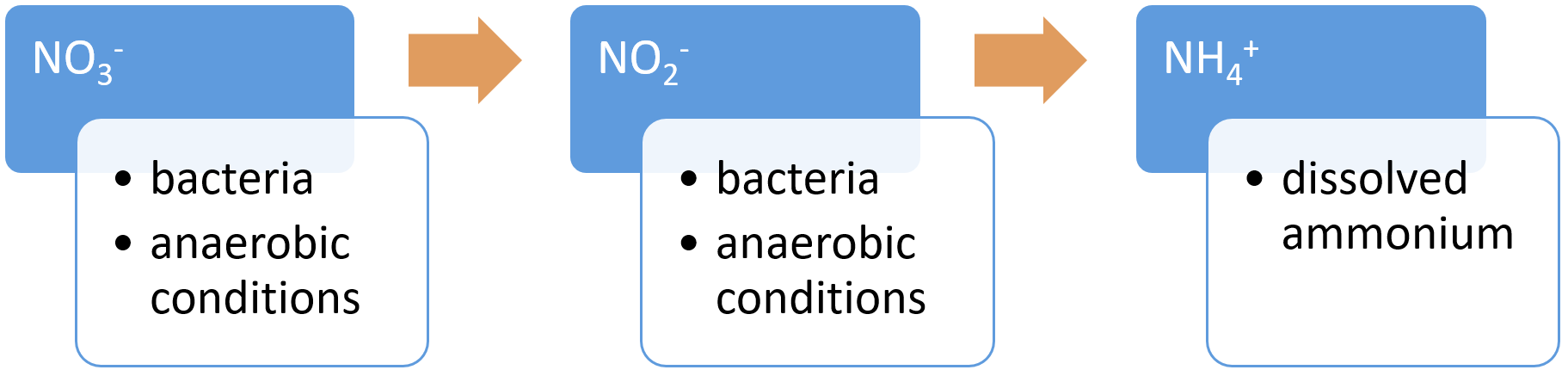
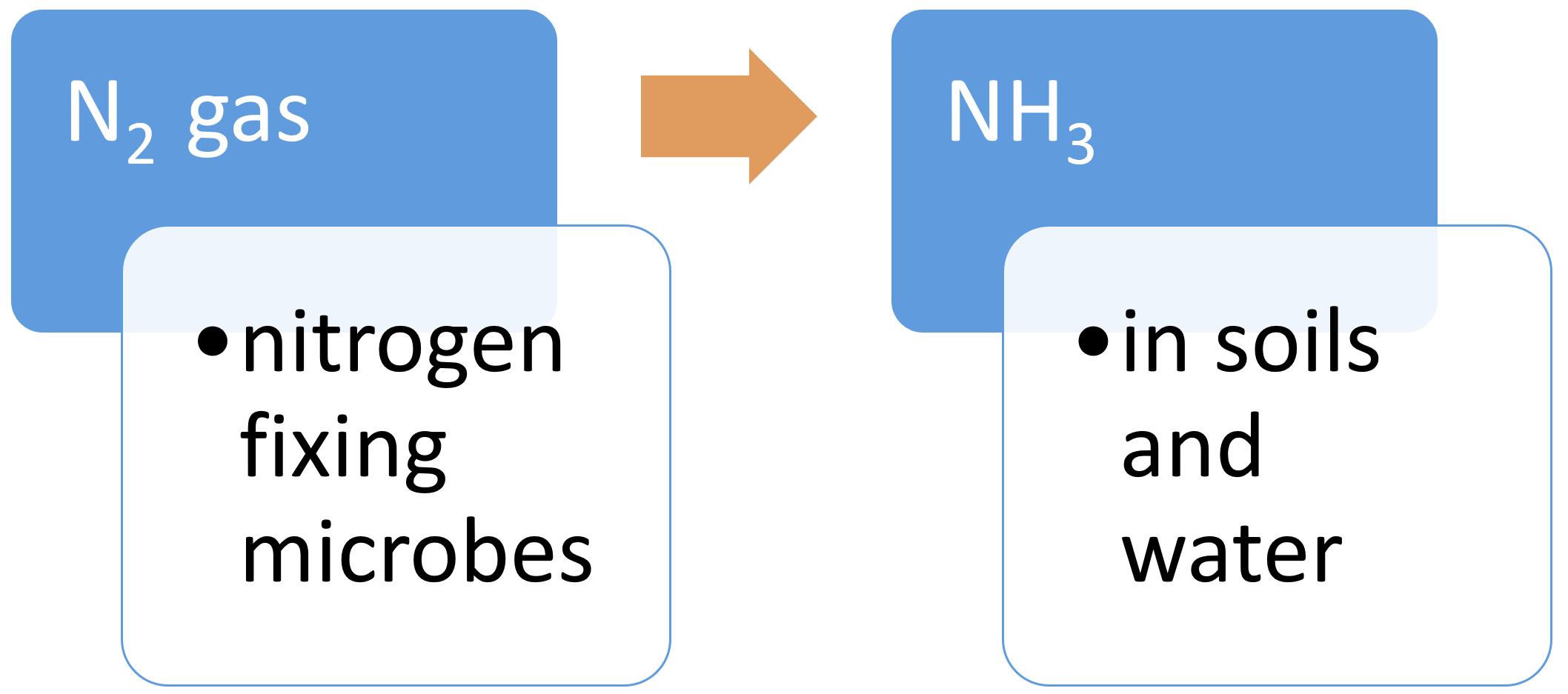
Dissimilatory nitrate reduction to ammonium (DNRA)DNRA is the reduction of nitrate (NO3−) via nitrite (NO2−) to ammonium (NH4+). DNRA can occur at the same time as denitrification, with each process preferentially favoured, depending on the availability of NO3− and the amount of organic carbon in the soil. In contrast to denitrification, DNRA does not contribute to N removal and retains N in the soil matrix[9]. DNRA occurs at a low soil redox potential (low oxidisation potential), under low NO3− conditions and high carbon (C) availability[4], with DNRA favoured over denitrification when the C to NO3− ratio is high[9]. Fixation (nitrogen)Nitrogen fixation is the conversion of nitrogen gas (N2) to ammonia (NH3), an ammonium ion (NH4), nitrate (NO3), or another nitrogen oxide[16]. These fixed forms of nitrogen can be used as a nutrient by living organisms. Fixation by microorganisms comprises more than 90 percent of all natural nitrogen fixation. There are two kinds of nitrogen-fixing microorganisms: free-living (non-symbiotic) bacteria, including cyanobacteria (or blue-green algae); and mutualistic (symbiotic) bacteria such as Rhizobium, associated with leguminous plants, and various Azospirillum species, associated with cereal grasses. Nitrogen is also fixed in nature as nitric oxide by lightning and ultraviolet rays[8].
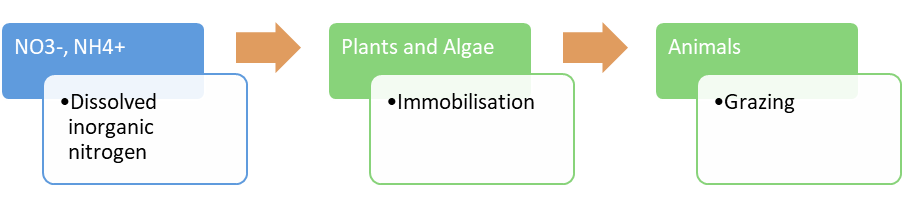
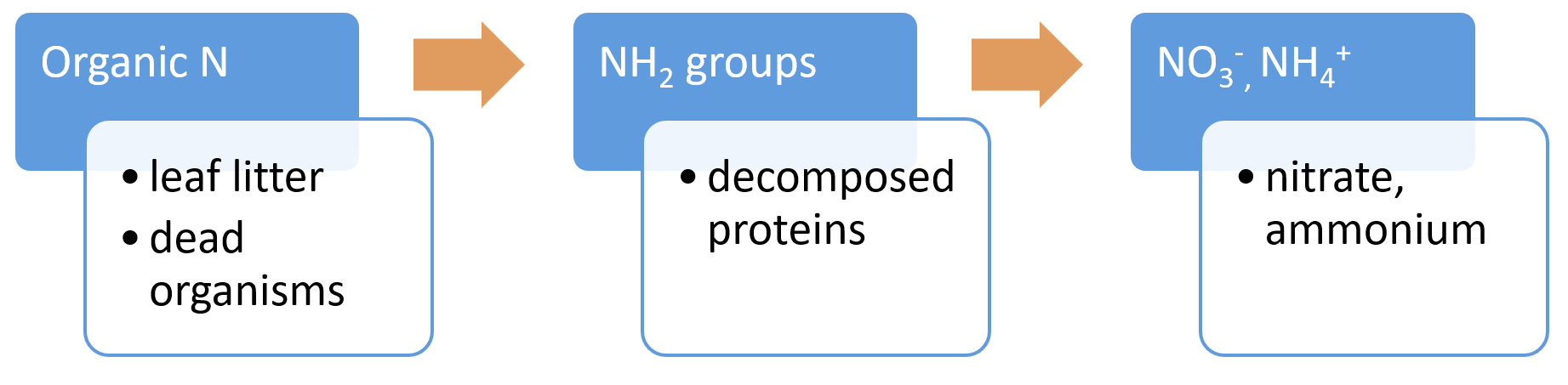
Food chain transfer (nitrogen)Nitrogen food chain transfer is the movement of nitrogen through food chains (food webs) by plants, microbes, invertebrates, fish, birds and other animals[10]. The amount that is transferred depends on factors such as the wetland type, the vegetation present, and the seasonal flooding regime. The nitrogen is processed internally through these food chains unless the animals/plants leave or enter the wetland which can result in nitrogen loss or gain to the system. Mineralisation (nitrogen) - (Decomposition/Ammonification)Nitrogen mineralisation is a two-step process comprised of a decomposition step, followed by an ammonification step. The decomposition process is the leaching of soluble compounds, physical fractionation, microbial conditioning (fungi and bacteria colonise leaf litter which alters the leaf substrate, and increases palatability), and feeding by invertebrates[12]. This can occur in the sediment (benthos) or as organic matter that is transported downstream. The rate at which organic N is processed depends on the chemical and physical properties of the organic N, the biota and environmental factors (e.g. temperature, oxygen, and other nutrients). The ammonification process is any chemical reaction in which NH2 groups (part of amino acids) are converted into ammonia or its ionic form, ammonium (NH4+), as a product[12].
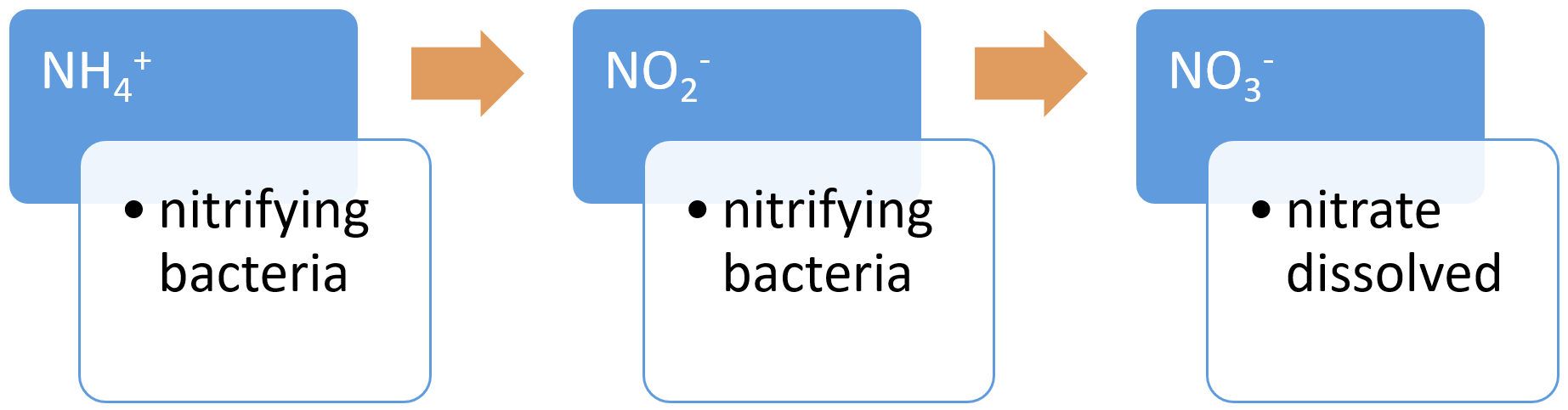
NitrificationNitrification is the biological oxidation of ammonium to nitrate under aerobic conditions[19]. It occurs in two forms: autotrophic nitrification and heterotrophic nitrification. Autotrophic nitrification, a process carried out by nitrifying bacteria, transforms ammonium (NH4+) in water and soil into nitrates (NO3−), which plants can incorporate into their tissue or biomass. Nitrification requires the presence of ammonium ions (NH4+), nitrifying bacteria (Nitrobacter and Nitrospira), a carbon source, oxygen availability, neutral pH, and temperatures between 20-30°C[20]. Heterotrophic nitrification is the transformation of organic N into nitrates (NO3−) by microorganisms, and occurs in acidic soils (pH less than 6)[23].
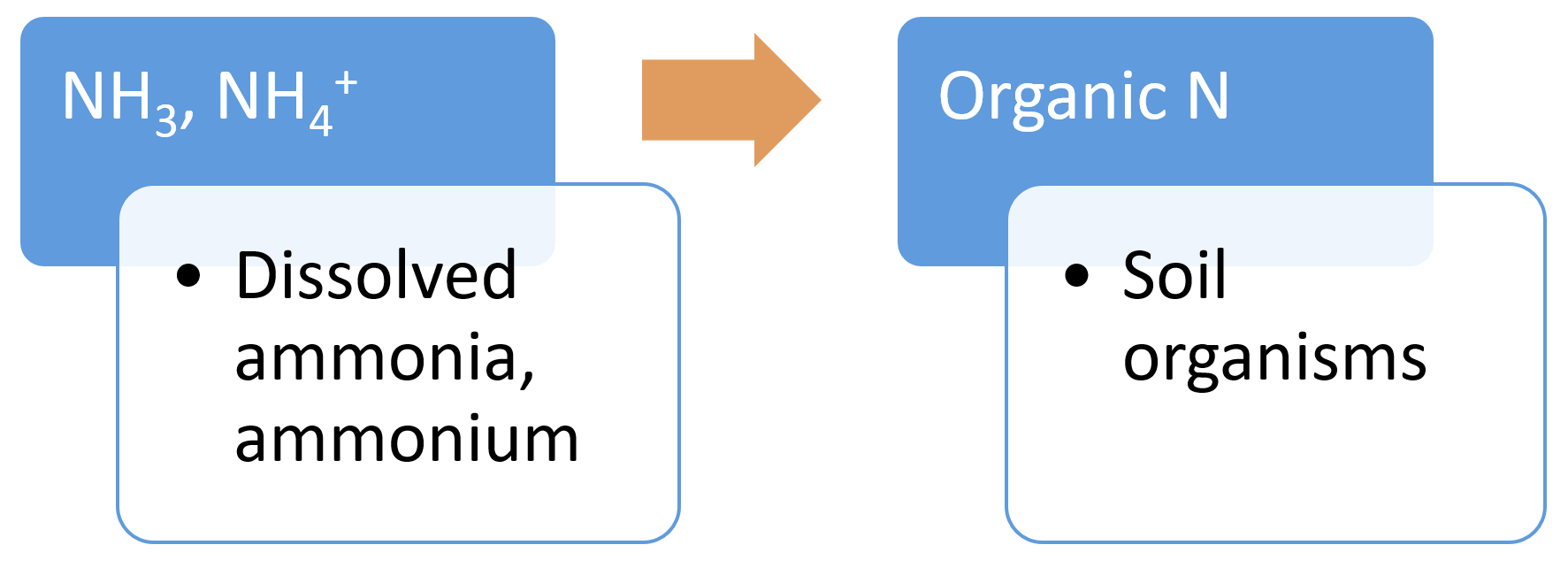
Immobilisation (nitrogen)Nitrogen immobilisation (assimilation) is to the long term storage of nitrogen in soil organic matter[13]. Nitrate and ammonium become unavailable to crops or other vegetation via immobilisation. Nitrogen immobilisation is the reverse of mineralisation.
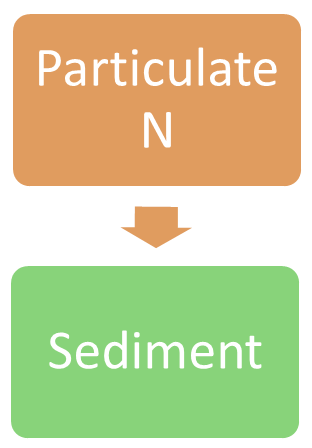
Sedimentation (nitrogen)Sedimentation is the process of depositing sediment. Sedimentation into wetlands typically occurs during large rainfall events and floods when it is eroded from the landscape. Nitrogen accumulated in the sediment in wetlands will be greater in areas receiving large suspended sediment loads, longer residence times, and slow water flows[8]. Sediment can play a role in carrying, storing and releasing particulate nitrogen. Depending on the wetland type and hydrology, some of the sediment may be re-mobilised (resuspended)[5].
References
Last updated: 31 July 2021 This page should be cited as: Department of Environment, Science and Innovation, Queensland (2021) Nitrogen processes and cycle – Processes, WetlandInfo website, accessed 8 May 2025. Available at: https://wetlandinfo.des.qld.gov.au/wetlands/ecology/processes-systems/nitrogen-concept-model/processes.html |

 — Department of the Environment, Tourism, Science and Innovation
— Department of the Environment, Tourism, Science and Innovation