|
|
PalustrinePalustrine – Processes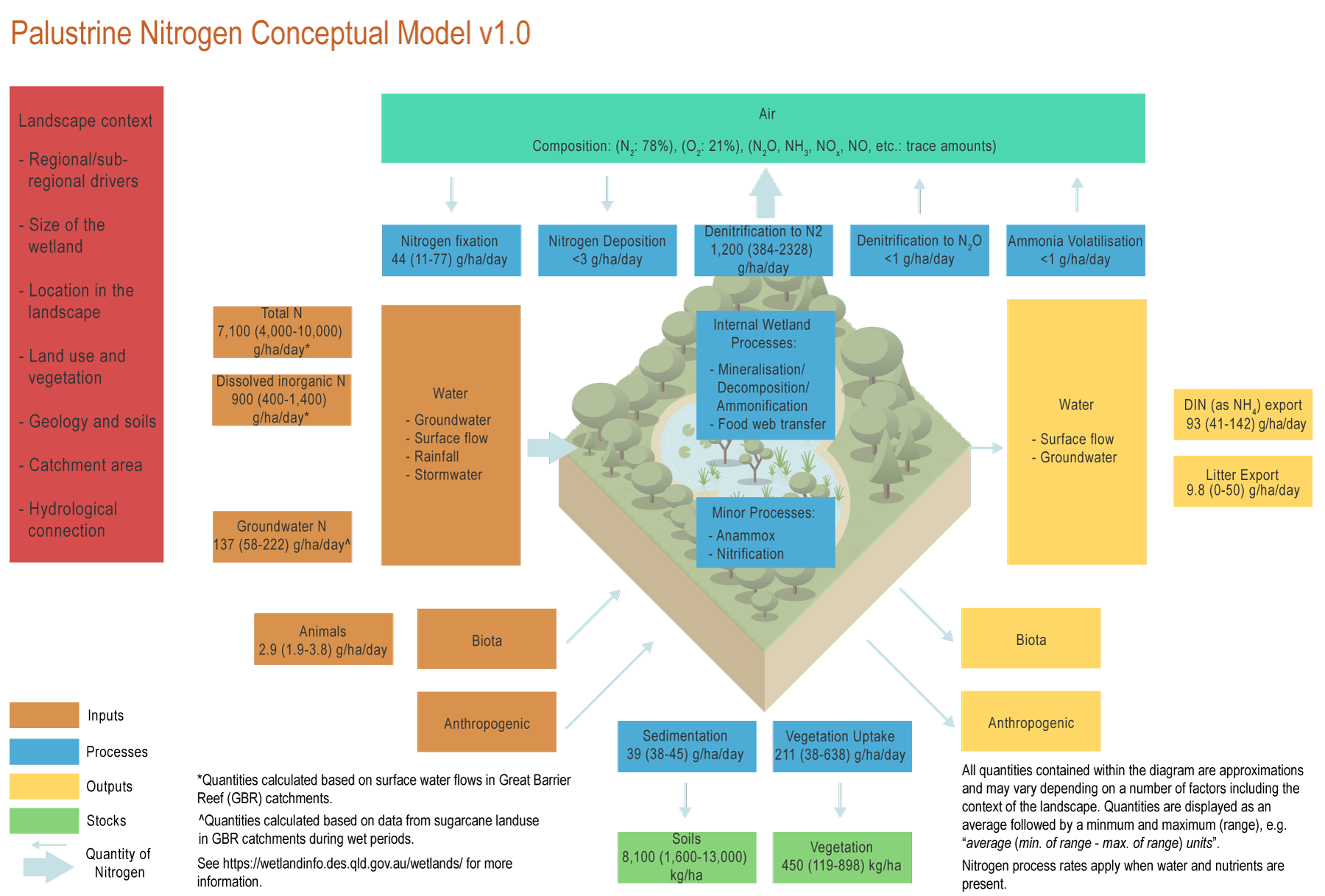
The conceptual models were compiled by researchers in collaboration with a wide range of stakeholders from Natural Resource Management groups, universities and government agencies and based on available scientific information[4]. Click on elements of the model or select from the tabs below Palustrine wetlands are considered hotspots of nutrient cycling due to their water logged and organic rich soils[11]. Nitrogen (N) can be transformed within palustrine wetlands through nitrification-denitrification, ammonification, mineralisation, immobilisation, biomass accumulation and sedimentation[11]. AnammoxAnammox (anaerobic ammonia oxidation) is likely to be a minor process in palustrine wetlands[9][14]. Biomass accumulation (vegetation)Nitrogen in palustrine wetlands is accumulated in plant material (biomass), through grass, herb, sedge, shrub, aquatic vegetation, and tree growth. Tree growth can accumulate around 211 (38-638) gN/ha/day*[2]. Grass accumulates about 7 gN/ha/day[2], but most of this N will return to the soil when the plant dies and is rapidly decomposed. Higher nitrogen accumulation as plant biomass will occur in palustrine wetlands with warm and wet conditions, and in areas with excessive weeds[2]. After dying, the macrophytes (including invasive weeds) will release N, which they have stored in their tissue[10]. Mineralisation (Decomposition/Ammonification)Decomposition in most palustrine wetlands, especially in deep soils (greater than 10 cm), is likely to be low due to the waterlogged and anoxic conditions, low pH, and toxic compounds of Melaleuca leaves[12]. Soils in palustrine wetlands have carbon:nitrogen ratios of around 14:1, suggesting that surface soils have favourable conditions for organic matter decomposition[2] which would result in NH4 production and a build-up of N in the soil due to mineralisation[5]. Deeper soils have conditions that favour accumulation of nitrogen. DenitrificationDenitrification in palustrine wetlands is usually high compared to other land types and is associated with the amount of nitrate available[1]. Denitrification in palustrine wetlands is also higher in soils rich in carbon (generally more than 5%), when temperature is between 20-30˚C and soil redox is between -100 to 200 mv[11]. Denitrification can account for 64-70% (1,200 (384-2328) g/ha/day)*[1] of N removal from palustrine wetlands. Export of nitrogen as N2 gas is likely to occur as a result of incomplete denitrification and nitrification processes, and typically accounts for less than 1% of nitrogen transformed by denitrification[4]. Food chain transferIn forested palustrine wetlands, light limitation and a source of carbon (e.g. Melaleuca or Eucalyptus leaves), which are toxic to most organisms, could mean relatively low transfers of N through the food chain (food web). In grass/sedge palustrine wetlands, epiphyton – especially if dominated by diatoms – could be transferred through the food web into insect, fish and bird biomass[8]. Palustrine wetlands that are frequently flooded have higher N transfer through the food chain as animals move into these wetlands to feed during the wet season[8]. NitrificationNitrification is limited in palustrine wetlands as they are generally anoxic and acidic (low pH). If wetlands are polluted and soils become anaerobic (no oxygen), nitrification will not occur, and most N will be in reduced forms (e.g. NH4)[11]. Nitrification is important for denitrification if N inputs are in the form of ammonium, as it transforms the N into the nitrate form required by denitrifying bacteria as an oxygen source. Nitrogen deposition from the atmosphereNitrogen can be deposited from the atmosphere to the biosphere as gas, dry deposition and precipitation. However, N deposition is only significant in areas with high industrial activity[13]. In the Great Barrier Reef catchments, N deposition is likely to be below 3 g/ha/day*[13]. Nitrogen fixation from the atmosphereNitrogen fixation from the atmosphere is highly spatially and temporally variable in wetlands. This fixation tends to be low at a rate of 44 (11-77) g/ha/day*, especially in sites where N inputs are high, such as in many wetlands within the Great Barrier Reef catchments[7]. N fixation tends to be high where macrophytes (aquatic plants) are present and nitrogen is limited[7]. SedimentationPalustrine wetlands usually receive sporadic amounts of sediment, especially during large floods. Nitrogen accumulated in the sediments of palustrine wetlands can be high in areas associated with large suspended sediment input loads, long residence times, and slow water flows[6]. Rates of sedimentation in palustrine wetlands are 39 (38-45) g/ha/day* or about one millimetre per year in floodplain wetlands in some catchments of the Great Barrier Reef[3]. Ammonia volatilisationAmmonia volatilisation occurs in basic conditions (higher than pH 8) and where concentrations of urea (derived from animals) are relatively high[13]. In palustrine wetlands, ammonia volatilization is likely to be minor (< 1 g/ha/day) as soils are usually acidic (less than 6 pH)[2]. *Nitrogen quantities are displayed as an average followed by a minimum and maximum (range), e.g. “average (min. of range - max. of range) units”. References
Last updated: 31 July 2021 This page should be cited as: Department of Environment, Science and Innovation, Queensland (2021) Palustrine – Processes, WetlandInfo website, accessed 8 May 2025. Available at: https://wetlandinfo.des.qld.gov.au/wetlands/ecology/processes-systems/nitrogen-concept-model/palustrine/processes.html |

 — Department of the Environment, Tourism, Science and Innovation
— Department of the Environment, Tourism, Science and Innovation

