|
|
Mangroves and the Carbon CycleMangrove forests occur in tidal waters and are some of the most carbon-dense forest types on earth[7]. Mangrove forests fix, transform, store, accumulate and export carbon via known and emerging carbon processes[3]. They are also critical blue carbon ecosystems. Quick facts
Mangrove carbon inputs are mainly through gaseous exchange as carbon dioxide (CO2) which is fixed by the trees through photosynthesis, producing wood, litter, and roots. Other inputs include tidal and freshwater flooding, which transports sediment, particulate organic carbon (POC), dissolved inorganic carbon (DIC) and organic carbon (DOC) into the forest. This carbon is then transformed to plant material, which can then be consumed through herbivory, decomposed by microbes, or stored as wood or sediment. The carbon that is not stored or consumed can be exported through the tides as dissolved carbon or as gaseous CO2 and methane (CH4).
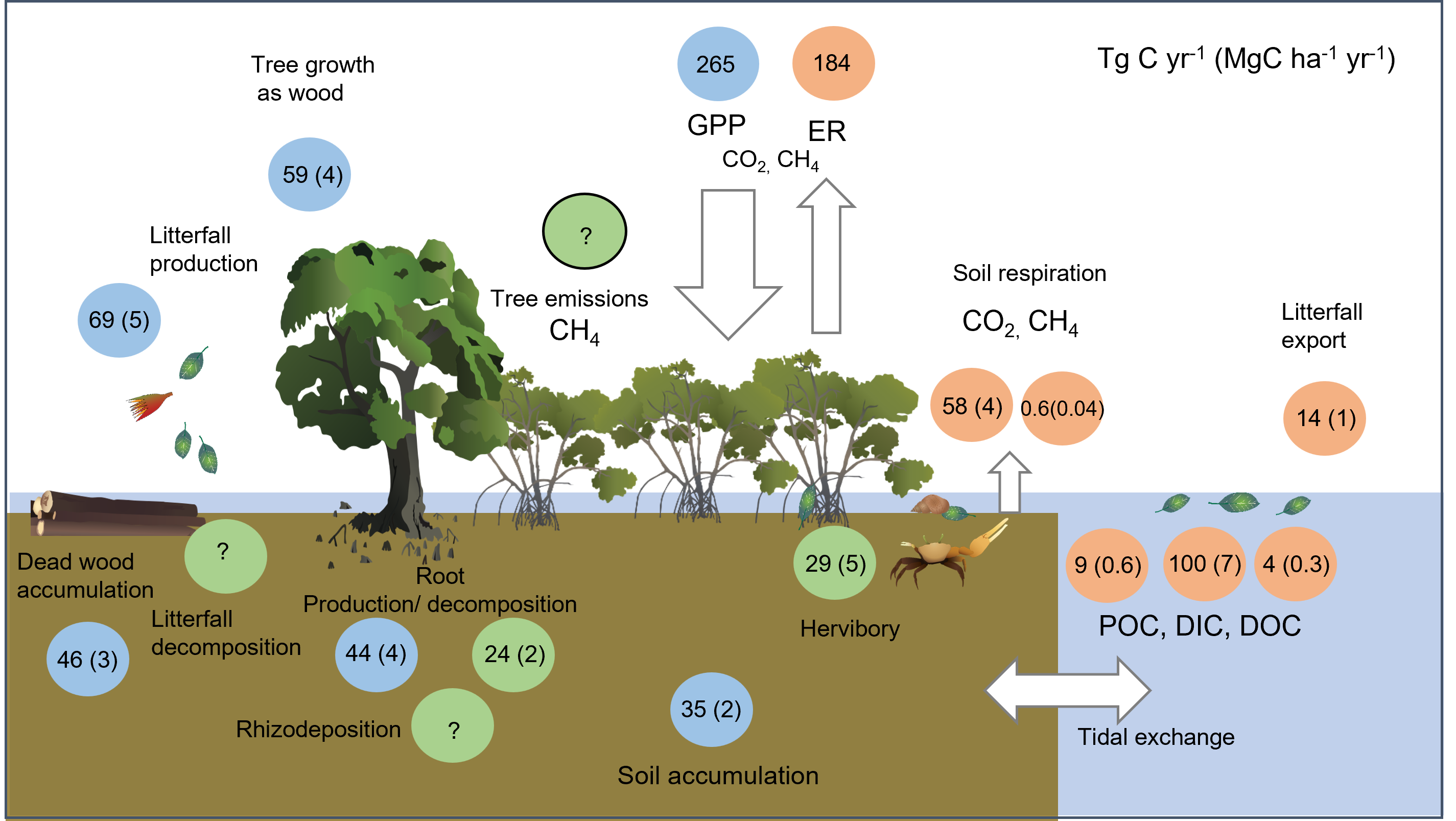
Figure 1 - Global estimates of mangrove fluxes listed as exchange (in teragrams of carbon per year) and per area (in megagrams of carbon per hectare per year); blue circles are gains, orange circles are losses, and green circles are transformations. The data used to calculate these global rates was obtained from Bunting et al. (2022) (14,735,899 ha). DIC, dissolved inorganic carbon; DOC, dissolved organic carbon; ER,ecosystem respiration; GPP, gross primary productivity; POC, particulate organic carbon. Adapted from Adame et al., 2024[3].
Carbon Accumulation (sequestration)Woody growthMangroves accumulate carbon in their trunks as wood, with global averages of 78.7 MgC (megagram or tonne) for every hectare of mangrove forest. Mangrove trees accumulate carbon as wood at a rate of 4.0 (0.13 – 21.8) MgC ha-1 yr-1[24]. Mangroves accumulate carbon as they grow and increase the size of their trunks, which occurs at an average of 0.31 cm every year, ranging from 0.0 to 1.84 cm[24]. The variation in carbon storage and growth in mangrove forests is mainly explained by variations in regional climate and tidal inundation[21]. LitterfallLitterfall production is the input of decaying leaves, stipules, flower parts, propagules and small branches. Leaves account for most of the litter, although large propagules of some species can be an important contributor. For example, the fruit of the “cannonball” mangrove (Xylocarpus granatum) can be as big as 12 cm in diameter. Mangroves produce litter year-round, however, the volume tends to vary seasonally. The highest litterfall production occurs in regions with mean annual temperatures above 25°C. Short and episodic disturbances such as tropical storms can also affect litterfall production[12]. Litterfall tends to be higher in riverine mangroves and lowest in lagoonal or carbonate settings, with global mean production rates of carbon as litterfall of 4.7 ± 0.1 (0.05 – 13.9) MgC ha–1 y–1[3]. DeadwoodDeadwood in mangroves can be classified as standing dead wood and as “downed wood”, which is wood on the forest floor. The contribution of deadwood to the carbon stored in mangrove forests is generally small (< 2%). The contribution to the mangrove deadwood stock in mangroves is 8.4 ± 12.7 MgC ha-1 for standing wood, and 15.0 ± 18.4 MgC ha-1 for downed wood[16], and rates of accumulation are 3.2 (1.6 - 5.7) MgC ha-1 yr-1[16]. Root growthIn mangroves, root production includes aboveground aerial and belowground roots, accounting for almost a third of the total production[4]. Mangrove fine roots (< 5 mm) store 3.0 ± 0.8 (0.2 – 12.1) MgC ha-1 yr-1, with variability explained by geomorphic settings, air temperature and precipitation, nutrients, salinity and species, although global trends are not clear[6]. Additionally, root exudates (secretions) in mangroves could contribute to the soil carbon of mangrove forests and increase the amount of dissolved organic carbon in the porewater. Soil/sedimentThe most extensive and permanent carbon stocks in mangrove ecosystems are in their soils/sediments, where organic carbon can remain for centuries or even millennia[2]. Carbon stocks in sediments are significantly lower in deltas of large rivers and higher in carbonate settings, while carbon sequestration is lower in areas without riverine inputs and highest in riverine mangroves and tidal systems[11]. This apparent contradiction is due to the sediment type deposited in mangroves at riverine settings, which has low carbon content, diluting the carbon concentration. Mean soil/sediment carbon sequestration rates are 2.3 (0.1– 6) MgC ha-1 yr-1.
Carbon transformationsHerbivoryConsumption of dead or decaying leaves (senescent), propagules and wood is primarily by crabs[14] but other animals such as insects and caterpillars can also consume mangrove leaves. Consumption of carbon within the fallen leaves is 2.0 ± 1.1 (0.3 - 7.2) MgC ha-2 yr-1[3] with the highest values found in mangroves where sesarmid crabs are abundant, where they can consume up to 87% of the total litterfall of the forest floor[14]. Root and leaf decompositionDecomposition is the breakdown of organic tissue, resulting in the loss of organic material as gaseous carbon (carbon dioxide, CO2, methane, CH4) or particulate or dissolved carbon. These chemicals can then be exported to tidal waters. Organic matter decomposition is highest when temperatures exceed 25°C[18] and lowest when litter contains toxic compounds, such as phenols[8] or is poor in nitrogen[22]. The decomposition of leaves is faster than that of roots and wood, especially when not buried in the soil[22]. Decomposition decreases with increasing salinity and root size, and varies among species[9]. Although data is scarce, root decomposition averages 1.6 (0.06 - 14.1) MgC ha-1 y-1.
Carbon LossesLateral carbon exportCarbon in the forms of litter, particulate and dissolved carbon, is exported from mangroves during ebb tides. Very large materials, such as branches and trunks of trees, can also be exported due to strong weather events, such as tropical storms[12]. Litterfall is usually exported in higher quantities in mangroves of arid climates[1]. Organic carbon export as particulates varies with tidal range, geomorphology, watershed size, and marine currents[20][23]. The export of dissolved inorganic (DIC) and organic carbon (DOC) is produced from the decomposition of organic matter. Dissolved inorganic carbon can be exported as carbonate alkalinity and partial pressure of carbon dioxide (pCO2), with the former increasing the buffer capacity of the ocean[19]. In contrast, the exported pCO2 can be outgassed back to the atmosphere or form carbonic acid, acidifying surrounding waters. Globally, mangroves export 0.27 ± 0.88 MgC ha-1 y-1 of DOC, ranging from an import of 0.67 to an export of 1.4 MgC ha-1 y-1[1]. Lateral DIC export is an order of magnitude higher, ranging an import of 4.2 to an export of 46 MgC ha-1 y-1, with a global average of 6.8 ± 2.1 MgC ha-1 y-1 and median of 3.6 MgC ha-1 y-1[19]. It is worth noting that data on DIC export is scarce and highly variable. RespirationSoil/sediment respiration involves the release of gaseous carbon (CO2 or CH4) from the soil/sediment due to the chemical oxidation of organic matter and the respiration of live roots, microbes and fauna[15]. Soil respiration varies due to differences in temperature, humidity, vegetation, soil microbes and fauna and the soil carbon-to-nitrogen ratio[17]. The soil CH4 fluxes in mangroves decrease with salinity while increasing with organic matter content[5]. Plants can also enhance CO2 and CH4 fluxes from the soil, for instance, through the branches and roots in Avicennia marina[10]. Finally, crab burrows can also facilitate CH4 efflux[13]. Globally, mangrove mean soil respiration is 3.9 ± 1.1 (2.2- 9.6) MgC ha-1 yr-1 of CO2 and 0.04 ± 0.19 (0.06 to 0.86) MgC ha-1 yr-1 of CH4. References
Last updated: 6 May 2025 This page should be cited as: Department of Environment, Science and Innovation, Queensland (2025) Mangroves and the Carbon Cycle, WetlandInfo website, accessed 8 May 2025. Available at: https://wetlandinfo.des.qld.gov.au/wetlands/ecology/processes-systems/carbon-cycle/mangroves-carbon-cycle/ |

 — Department of the Environment, Tourism, Science and Innovation
— Department of the Environment, Tourism, Science and Innovation